
Our pre-clinical and clinical projects aim to provide in vivo metabolic characterization of PPGLs in relation to their genetic alterations using magnetic resonance spectroscopy imaging and 13C-Glucose metabolic tracing approaches.
Proton Magnetic Resonance Spectroscopy (1H-MRS) is a special magnetic resonance imaging sequence used to identify specific metabolites linked to mutations found in PPGLs.
In particular, in patients with a PPGL linked to a mutation in an SDHx gene, there is a complete loss of function of succinate dehydrogenase (SDH, an enzyme in the TCA cycle that oxidizes succinate to fumarate), resulting in massive accumulation of succinate in the tumor. We have previously used 1H-MRS to identify succinate in PPGL tumors in vivo and demonstrated that this metabolite is a non-invasive biomarker specific for SDHx mutations. Thanks to this same technique, we were also able to demonstrate an indisputable causal link between the development of pituitary adenomas and the presence of an SDH mutation.)
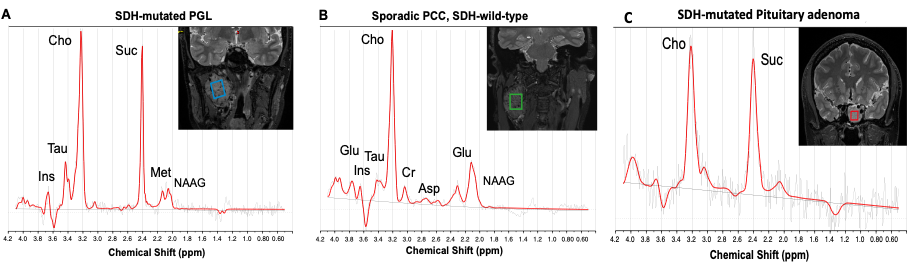
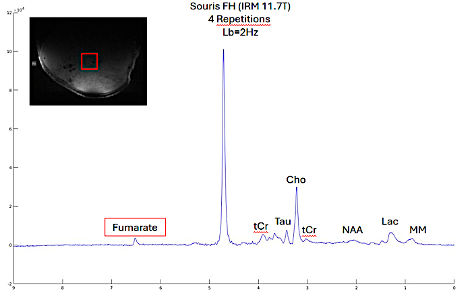
Our hypothesis is that most genetic mutations predisposing to PPGL have an impact on metabolism, with a specific metabolic profile detectable by 1H-MRS. To this end, we are currently studying the specific metabolic profiles associated with 4 mutated genes (Sdhb, Scl25a11, Fh and Vhl) in mouse allograft models on the 11.7T small animal MRI of the CENIR (Center for Neuroimaging Research – Paris Brain Institute). An accumulation of fumarate has already been observed in the Fh
We also plan to develop a specific diagnostic test for SDHx mutations, enabling rapid identification of patients using a metabolic flux analysis approach based on the use of a stable glucose isotopomer (13C-Glucose). We have recently demonstrated in a cell model that cells deficient for the Sdhb gene exhibit a significant metabolic “switch”, leading in particular to the specific production of an aspartate isotopomer. The hypothesis of our translational research is that this major deficit in glucose metabolism is present in patients with SDHx-mutated PPGL. Thus, using the same metabolic flux approach, intravenous (IV) administration of a single dose of 13C6-glucose would enable us to identify very quickly and simply the presence of an SDHx mutation in a PPGL patient. This project is in the process of obtaining the necessary technical and regulatory authorizations.

