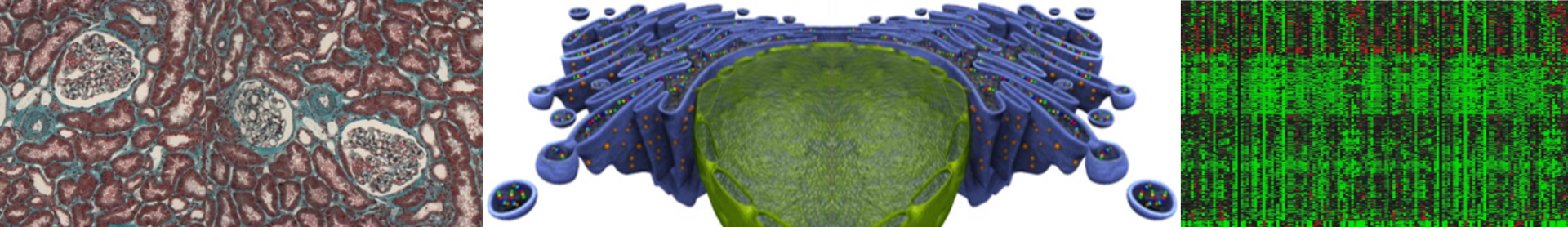
End-stage renal disease (ESRD) is a medical condition resulting from chronic kidney disease (CKD), which affects millions of persons worldwide and constitutes a major public health problem and economic challenge. Despite considerable progress, numerous challenges remain related to the mechanisms, the prevention and the cure of CKD, with immediate consequences for the patients in terms of mortality, morbidity and cost to society. The renal medicine field needs to improve the current knowledge of the molecular processes that are critical for renal disease establishment and progression, and to accelerate the translation of basic science discoveries in the clinical field in terms of both early diagnostic and therapeutic strategies. We are willing to provide mechanistic insights into how the injured kidney activates adaptive responses to stress at the cellular level that would lead to CKD from in vitro cell models to genetically engineered murine models, transferring their clinical consequences to large-prospective human cohorts.
Acute Kidney Injury is associated with micro-environmental alterations and homeostasis disturbances, forcing cells to activate biological processes leading to profound metabolic reprogramming, which promotes cell survival in the injured milieu and eliminates stressors. In turn, disease reflects the inability of adaptive responses to restore tissue homeostasis. Kidneys have to cope with a wide array of insults that translate into elementary stressors at the cellular level (e.g., nutrient starvation, hypoxia, oxidative stress, inflammatory stress, and proteostasis disturbances). Adaptive responses to these stresses are often evolutionarily conserved molecular systems that primarily aim to eradicate or reduce stress intensity and promote metabolic reprogramming to maintain cellular homeostasis and other vital functions. Molecular modules sense microenvironmental fluctuations in nutrients, oxygen, and temperature, as well as disordered intracellular fluctuations, such as the accumulation of unfolded proteins and energy (ATP) shortage, among others.

These modules then transduce signals that will fuel metabolic reprogramming to maintain basal functions, while adapting the cell to the new environmental conditions. In addition to cellular decisions of life and death, these adaptive responses also participate in building communication networks that shape the stressed cell microenvironment, generally in a paracrine manner, leading to the activation of preemptive responses in cells that have not yet been subjected to the stress and to the production of alarm signals. Therefore, adaptive stress responses pathways are likely critical for tissue remodeling, as they shape the endogenous repair and scarring equilibrium in tissues and therefore significantly impact the functional outcomes of the injured kidney, ultimately leading to CKD. In addition, these molecular reprogramming circuitries and cellular adaptive responses occur very early after the initiation of AKI, well before cell death and the engagement of the maladaptive repair process. Thus, the detection of their activation constitutes an opportunity for an early diagnosis of ongoing tissue injury. Hence, characterizing the molecular mechanisms underlying the cellular responses to acute stress and their structural and functional consequences at the tissue level is crucial for the development of preventive and therapeutic strategies in renal medicine.
There is now clear evidence that ER stress or parts of the UPR that, for instance, target epithelial cells actively participate in the development of AKI and CKD, and we are currently developing a research program designed to demonstrate that the UPR, engaged upon acute tissue injury, is critical for tissue remodeling and therefore significantly impacts the functional outcomes of the injured kidney.
In summary, our projects have the goal of filling the gap between basic science and applied biomedical research and providing clinicians with accessible tools for the early prediction of CKD evolution, offering them the possibility to better personalize clinical management and treatment.
Publications :
Taurine Deficiency Is a Hallmark of Injured Kidney Allografts. A. Rinaldi, P. E. Cippà, I. Nemazanyy, D. Anglicheau, N. Pallet. Transplantation, 2024, Vol. 108 pp. e218–e228 Link.
STAT3 Drives the Expression of ACSL4 in Acute Kidney Injury. V. Poindessous, H. Lazareth, G. Crambert, L. Cheval, J. L. Sampaio, N. Pallet. iScience, 2024, Vol. 27 pp. 109737 Link.
Lipidomic Profiling of Kidney Cortical Tubule Segments Identifies Lipotypes with Physiological Implications. L. Cheval, V. Poindessous, J. L. Sampaio, G. Crambert, N. Pallet. Function (Oxford, England), 2024, Vol. 5 pp. zqae016 Link.
Impaired Fatty Acid Metabolism Perpetuates Lipotoxicity along the Transition to Chronic Kidney Injury. A. Rinaldi, H. Lazareth, V. Poindessous, I. Nemazanyy, J. L. Sampaio, D. Malpetti, Y. Bignon, M. Naesens, M. Rabant, D. Anglicheau, P. E. Cippà, N. Pallet. JCI insight, 2022, Vol. 7 pp. e161783 Link.
Validation of a Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for Simultaneous Quantification of Tryptophan and 10 Key Metabolites of the Kynurenine Pathway in Plasma and Urine: Application to a Cohort of Acute Kidney Injury Patients. Z. Nadour, C. Simian, O. Laprévote, M.-A. Loriot, I. A. Larabi, N. Pallet. Clinica Chimica Acta; International Journal of Clinical Chemistry, 2022, Vol. 534 pp. 115–127 Link.
Cell Stress Response Impairs de Novo NAD+ Biosynthesis in the Kidney. Y. Bignon, A. Rinaldi, Z. Nadour, V. Poindessous, I. Nemazanyy, O. Lenoir, B. Fohlen, P. Weill-Raynal, A. Hertig, A. Karras, P. Galichon, M. Naesens, D. Anglicheau, P. E. Cippà, N. Pallet. JCI insight, 2022, Vol. 7 pp. e153019 Link.